I’ve been through a long history of cholesterol wars – how bad is it and could it even be good?
In this article, I’m going to offer my opinion, which is a balanced, undogmatic, and a middle path in the debates.
This is one of the most important topics in health and longevity because cardiovascular disease is the biggest killer in the West, and therefore can have a big impact on longevity.
This is also a topic that people cling to with religious fervor, so I will likely get into trouble for these opinions, but they are what I believe, and backed by my readings over the years.
On the one hand, you have Carnivore enthusiasts, who say lipids have nothing to do with cardiovascular disease.
On the other hand, you have people like Peter Attia, who is a strong proponent of getting cholesterol down as low as possible to prevent cardiovascular disease and thinks there are no negatives to having almost no circulating cholesterol.
While there are some unsettled questions, I will describe how I see the current state of the evidence and how I act based on it.
As opposed to many others, my aim is to “steel man” or strengthen opposing positions before I disagree with them.
You will see that I am not here nor there with Carnivore or Attia. I take a more nuanced approach, and I’ll explain why.
Counterpoints to the Hypothesis that High Cholesterol Causes Cardiovascular Disease (CVD)
1) One counterpoint to the cholesterol/lipid hypothesis is that there are many other factors to CVD, including blood pressure, inflammation, insulin resistance, oxidative stress, etc…There’s no question that this is true, but there’s no reason to discount one of the causal factors – lipoproteins.
2) A second counterpoint is that there are better lipid indicators of cardiovascular risk than LDL-C – mainly APOB, triglycerides, VLDL, non-HDL-C, LP(a), LDL-P, small dense LDL-P, oxidized LDL, etc…
This may be true, but LDL-C still correlates pretty well with these other markers and is the most easily tested and studied biomarker.
However, I agree, it’s important to look at the whole picture and try to get more advanced tests than LDL-C, which is commonly provided.
3) Association is not causation. This is true, but there are many lines of evidence showing that cholesterol is not merely associated with cardiovascular disease, but also a causative factor.
It’s true that there are some correlative aspects of LDL cholesterol with suboptimal bodily function – including thyroid hormones, bile acid/toxin removal, insulin resistance, gut microbiome imbalance, etc…
For example, optimal levels of thyroid hormones lower cholesterol, but also lower cardiovascular disease risk in other ways [1].
In addition, bile acids in circulation can predict cardiovascular disease, and potentially not using cholesterol to create and excrete bile can increase risk [2, 3, 4]. Lower excretion of bile also means less toxin removal.
Higher LDL is associated with insulin resistance as well as being overweight, and many other unhealthy behaviors.
In any case, even if it’s merely an association that is linked with other health issues, optimizing the biomarker may improve other aspects of your health.
For example, I’ve come to realize that I should increase my thyroid hormone levels more and produce and excrete more bile – to optimize not only my cholesterol levels but overall bodily function.
4) People will say that there is evidence that in a specific age group, or if you slice the data in a very specific way, especially among older people, cholesterol can be beneficial. There’s some truth here as well, and I’d like to dig into this a bit more.
People in general are very uncomfortable with the fact that in biology, something can be both good and bad. People want clear villains and heroes. They want to understand something as good or bad, not as – well it depends and it’s complex.
LDL/APOB is one of those things that may be both good and bad. It’s clear that it’s bad for cardiovascular disease, but there’s some reason to believe that it’s good in other ways.
Peter Attia seems to think that there are practically no benefits to LDL/APOB in part because infants don’t seem to have any issues and they have much lower levels of lipids. I don’t necessarily agree with this logic, but the jury is still out on what the benefits are.
Potential Benefits of LDL & Cholesterol
As opposed to Attia and in agreement with some of the cholesterol skeptics, I think there are some benefits to cholesterol.
There are 3 main lines of evidence why I believe this.
- Mechanistic studies show various benefits of cholesterol.
- The most studied drugs or diets to reduce cholesterol don’t reduce the overall risk of death.
- Various association studies show that in the elderly, LDL can even be protective.
In a large systematic review, high LDL-C is actually associated with lower death risk in most people over 60 years [5].
Since cardiovascular disease is the biggest killer in the Western world, and it’s pretty clear that LDL causes CVD, then it would make sense that LDL has some benefits if the overall death rate doesn’t decline.
Attia says that the vast majority of the cholesterol used in the brain is produced and used locally, and circulating levels shouldn’t influence it much.
Compared to Attia, I tend to think there are probably more benefits to circulating lipids than we can prove at the current moment, and that’s the reason why some people evolved to have higher levels.
Here are some benefits of cholesterol:
- Cellular repair and maintenance: LDL cholesterol is essential for the proper functioning of cell membranes. It helps maintain their structural integrity and fluidity, which in turn supports overall cellular health.
- Helps hormone production – testosterone, estradiol & cortisol
- Helps produce vitamin D
- Helps produce bile acids to digest fat and absorb nutrients
- Helps intracellular transport (eg, for the fat-soluble vitamins A, D, E, and K)
- May play a role in mood via serotonin receptors & serotonin metabolism [6, 7, 8, 9].
- Binds certain toxins, making them unable to trigger harmful immune responses. LDL binds toxins produced by bacteria (Staphylococcus) and the toxin lipopolysaccharide (LPS) [10, 11]. This is why when people get systemic inflammation, those with higher LDL are more likely to survive.
- Helps repair damaged blood vessels. When arteries become damaged, LDL binds to the artery wall to aid the healing process. This helps the artery in the short term but can be harmful long-term [12].
- It’s necessary for synapse formation and myelin production – and therefore cognitive function. Cognitive dysfunction is a side effect of statin use, and I experienced it as well.
- Increasing T Regulatory Cells and thereby helping create tolerance that may help autoimmunity and food sensitivities [13, 14, 15, 16]. This may be why people who are more prone to autoimmunity like diets that increase their cholesterol.
- Muscle function & recovery – for example, HMB appears to be metabolized within skeletal muscle into cholesterol, which may then be incorporated into the muscle cell membrane, thereby enhancing membrane integrity and function [17]. Muscle pain is the most common side effect of statins, which I experienced as well.
12) Fighting infections – Most Significant?
I separated this one from the rest because it seems to be the most plausible mechanism by which higher cholesterol can protect against dying, especially in the elderly.
Cholesterol seems to play a role in helping the immune system fight infections (18, 19).
A large meta-analysis of 19 cohort studies including 68,406 deaths, found higher cholesterol was associated with lower death risk from respiratory and gastrointestinal diseases, most of which are of an infectious origin [19].
Respiratory infections are very common, and a significant cause of death in the elderly, as we’ve seen from the recent pandemic.
Men with low cholesterol had significantly fewer circulating lymphocytes, total T cells, helper T-cells, and CD8+ cells than high-cholesterol men. Also, phagocytic activity and spontaneous motility of mononuclear cells were significantly higher in high-cholesterol men [19].
Higher cholesterol also is associated with reduced risk of [19]:
- Being admitted to the hospital due to an infectious disease
- Risk of being admitted to the hospital because of pneumonia or influenza
- Severe and critical COVID-19 [20]
- A wide variety of infections
- Urinary tract infections
- HIV infection and the risk of death in AIDS
- Postoperative abdominal infections
- Adult chronic carriers of hepatitis B
It’s still not clear if these are causal or just an association. Cholesterol synthesis and clearance are increased in infection and can go up or down depending on the infection [19].
When people get older, they are more susceptible to dying from an infection, and that could be why LDL could be correlated to a lower risk of death in people over 60 – when people start to die from infections.
My father died from a respiratory infection pretty suddenly at the age of 66, and had normal cholesterol levels, while my grandmother lived to 93 with high cholesterol her whole life and eventually died from cardiovascular disease – but it took a while.
This is obviously just 2 anecdotes, but highlights what could be going on at a population level. Lower your cholesterol and reduce your risk of CVD, but increase your risk of dying from a respiratory infection – perhaps.
Similarly, cholesterol lowering drugs are generally given to people who are 60+. While cardiovascular disease goes down, overall death risk could be counteracted by other factors like infections, especially in the elderly.
U Shaped Curve of LDL Cholesterol
I decided to look at recent large studies of mortality in different populations to get a better idea of the most recent research on the subject.
Three large studies were done in the US, Denmark and Korea, all published in good journals [21, 22, 23].
They all came to the same conclusion – that there’s a U shaped curve in death rate for LDL/total cholesterol. Both low and high LDL cholesterol are associated with an increased risk of death.
In the USA study, after adjustment for age, sex, race and ethnicity, education, socioeconomic status, lifestyle factors, C‐reactive protein, body mass index, and other cardiovascular risk factors, individuals with LDL‐C <70 mg/dL, compared to those with LDL‐C 100–129.9 mg/dL, had a 45% higher risk of dying from all‐causes. Similarly, LDL cholesterol over 190 had a 49% higher risk of dying compared to the 100-129.9 group [21].
In a Danish study, compared with individuals with concentrations of LDL-C of 132-154 mg/dL), the multivariable adjusted risk for all-cause mortality was 25% higher for individuals with LDL-C less than 70 mg/dL and 15% higher for LDL-C of more than 189 mg/dL. The concentration of LDL-C associated with the lowest risk of all-cause mortality was 140 mg/dL in the overall population and in individuals not receiving lipid lowering treatment, compared with 89 mg/dL in individuals receiving lipid lowering treatment [22].
It also found that any increase in LDL-C levels was associated with an increased risk of heart attack [22].
In the Korean study of 12 million people, total cholesterol that was low or high also increased overall mortality. The lowest mortality was a total cholesterol of 210-249 [23]. They didn’t look at LDL-C, but it would correspond to somewhere around 105-140, which is in agreement with the other studies done on LDL-C.
Interestingly, they also found that lower cholesterol (around 20-40 mg/dl lower) was even better for people under 45 [23].
Lower Triglycerides is Better
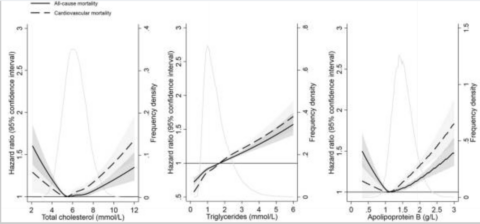
In an observational study looking at total cholesterol, ApoB and triglycerides, we see an interesting picture [24]. The solid line in the graph indicates total mortality.
You can see for total cholesterol and ApoB, the image confirms the other observational studies – a U-shaped curve. When we look at triglycerides, however, we see a different picture. It’s linear, which means the lower the better.
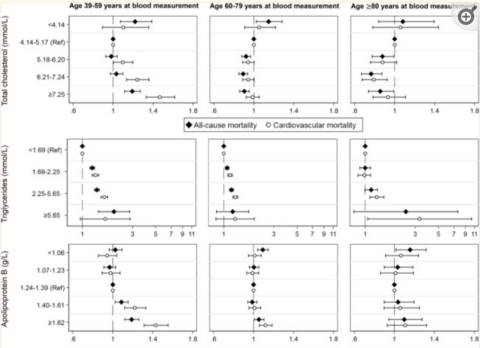
You can also see the curves changing a bit based on age. For people who are younger, you see higher mortality from higher cholesterol (39-59), whereas that’s not quite the case for older people – unless it’s very high.
For ages 39-59, the optimal range of total cholesterol is quite wide – from 160-279.
Triglycerides are age independent when it comes to risk, where you can see that for every age group the same situation occurs. What might be the case is that triglycerides don’t protect against infections or have other benefits like cholesterol, and therefore lower is better. Or it could just be significantly more damaging, which is commonly thought to be the case. Triglyceride levels are mostly lifestyle based, significantly more so than LDL.
Making Intelligent Decisions Under Uncertainty
It’s not completely proven that having higher blood levels of LDL/Cholesterol/Lipoproteins will result in better outcomes in any particular area.
However, the fact that many drugs that decrease cholesterol don’t result in a lower risk of overall death and that the best association studies show a U shaped curve for mortality, it points to potential benefits of cholesterol.
It seems like the mainstream recommendation of non HDL cholesterol of less than 130 or LDL-C of <130 could be an optimal range for cholesterol as a whole.
Approach 1: Intelligent Risk Reduction
It would make sense that specific people should focus on lowering cholesterol:
- People with a previous cardiovascular event
- People at high risk of cardiovascular disease (based on genetics, labs, and risk assessment)
- People with higher non HDL cholesterol (over 130) or ApoB or small dense LDL – since these are more directly related to CVD than LDL-C.
- Younger people under the age of 60
In this approach, you’re looking at what you’re most likely to die from and focusing on preventing that. Since 50% of people die from CVD, that is the big elephant in the room. If you are at higher genetic risk for CVD, then that is something that you should focus on more.
Approach 2: Start Young
The fact remains that high cholesterol predicts coronary heart disease in young and middle-aged men [19].
It could make sense for adults under the age of 60 to lower their cholesterol, because the buildup of plaque starts when you’re born.
When you’re young, there’s no significant risk of dying from an infection, so it would make sense to slow the plaque buildup when you’re younger, ideally without pharmaceuticals of course.
Approach 3: Biohacker Approach
Last is the biohacker approach. If you see yourself as an advanced longevity athlete such as myself, then it could make sense to lower LDL while also boosting your immune system and counteracting any other negative effects from lower LDL – including after the age of 60.
This is a much more complex approach of course, but I plan to follow it. In this approach, I look at all the potential downsides and counteract each one.
Given that I am young, I want to be even more aggressive. My goal for non HDL-C of <120 and an ApoB <90.
3 ways to check if you’re at high risk
- Using advanced polygenic risk scoring based on genetics to see your predispositions for atherosclerosis, coronary artery disease, strokes, heart attack, deep vein thrombosis, heart failure, atrial fibrillation, and other cardiovascular diseases. SelfDecode is the only consumer company that provides these cutting-edge predispositions.
- Looking at all of your labs related to heart disease predisposition – not only cholesterol. SelfDecode likewise makes this easy by listing all of the markers related to cardiovascular disease, the optimal ranges and recommendations to get them there.
- Looking at your lifestyle risk. SelfDecode has questionnaires that do exactly this and you can see what your environmental risk is, which can be independent of genetics and labs.
The Evidence that LDL-P and APOB Cause Cardiovascular Disease
The idea that LDL-P/APOB is not related to cardiovascular disease is almost certainly wrong, given the evidence available.
The lipid hypothesis is one of the most studied and proven theories in all of medicine, and there are many lines of evidence pointing in the same direction.
- Mechanistic studies – there are plausible mechanisms by which lipoproteins can cause heart disease by depositing in arteries.
- Animal studies – you can induce cardiovascular disease (CVD) in animals by increasing cholesterol
- Association studies – there’s a huge number of studies that show people with higher LDL-C and lipoproteins have a higher risk for CVD.
- Many drugs that lower LDL/APOB also lower death from cardiovascular disease: statins, PCSK9 inhibitors, ezetimibe (inhibits cholesterol absorption), niacin, bile acid sequestrants, fibrates, etc… All of these drugs work differently and all show effects in lowering some parameter of cardiovascular disease.
- Genetic studies – single mutations in LDL receptors, PCSK9, and other genes that cause very high cholesterol result in a higher risk for CVD.
- Mendelian randomization studies – higher genetically predicted levels of LDL or APOB or non HDL-C based on many variants are causally linked to CVD and death. This means that if you have a general genetic predisposition to higher levels of LDL-C or APOB, then that results in a higher risk of CVD. Since that’s an independent variable not based on lifestyle, it shows a causal association.
There are not many things in medicine where you have all of these 6 lines of evidence converging to the same conclusion.
Even HDL cholesterol, which for a long time was thought to causally reduce the risk for heart disease, turned out to not be causal. Drugs that increase HDL-C and mendelian randomization studies don’t show that increased HDL causally reduces risk – it seems to be just an association.
Drugs/Diets that Lower Cholesterol: Reduced Overall Death Risk?
To understand whether lipids have a causal impact on cardiovascular disease, it helps to look at drug studies.
There are a wide variety of classes of drugs that lower cholesterol, with unique mechanisms of action, that all show the same thing – lower cardiovascular disease risk when people take the drug.
Plant-based diets also lower cholesterol and correlate with lower CVD risk. As opposed to drugs, however, there are mainly association studies for CVD and plant-based diets.
In terms of overall mortality statistics, statins are the only drug that shows a decrease in the overall death rate, whereas the other classes of drugs don’t show any change in overall mortality.
Statins are also the only of these drugs to decrease inflammation & combat infectious disease, which seem to play a role in the mortality decrease.
It wasn’t clear 20 years ago that this was the case, because most of the studies came out in the last 8 years.
As time goes on, more and more data has become available on all of these drugs with regard to their effect on CVD and overall death.
Statins
Statins are a class of drugs that lower not only cardiovascular death but also overall mortality and are proven time and again to do so (at least when given to people at higher risk) (25).
However, the confounding variables are that statins can lower inflammation, which we know is a significant mechanism of heart disease [26].
Statins also have antioxidant effects, prevent blood clotting and combat infectious diseases caused by viruses, protozoa, fungi, and bacteria [26].
Statins are associated with a lower risk of viral infections [27].
Indeed, association studies show that statins have a protective effect on infection-related mortality [28].
Not surprisingly, prior statin use is associated with lower risks of mortality or severe disease in patients with COVID-19 [29].
Along the same lines, statins are just as effective whether cholesterol is lowered by a small or large amount (by more than 40%). In addition, statin treatment is effective whether the initial LDL-C is high or low [19].
When we look at other drugs that lower cholesterol, they seem to reduce cardiovascular risk, but not overall death risk – and that’s because they don’t also have the other positive effects of statins.However, the side effects of statins are real and can be significant. I’ve tried a statin and felt all of the known side effects – serious muscle pain and a decline in motivation and brain function. I’ve tried 3 different statins and all of them had similar side effects. I, therefore, don’t take them.
Keep in mind that statins have a selection bias. Only people who are at high risk are generally taking statins and people who experience side effects likely don’t take them.
PCSK9 Inhibitors
PCSK9 inhibitors are a new class of drugs that dramatically lower cholesterol.
I’ve taken a PCSK9 inhibitor (Evolocumab) twice to try it out, and actually I didn’t experience any noticeable side effects – as opposed to statins, and they were quite powerful in bringing down cholesterol.
I recently looked at the CVD risk reduction of PCSK9 inhibitors and the overall death (mortality) risk.
I looked at the most recent meta-analyses, published in December 2022. The results are as follows [30]:
“We incorporated 12 RCTs with 53 486 patients total, of which 27 674 received PCSK9 inhibitors and 25 812 received placebos. The mean follow-up duration was 1.56 years. The effect of PCSK9 inhibitors on major adverse cardiovascular events (MACE) was statistically significant, and the corresponding mean NNT was 36. Alirocumab reduced the risk of MACE, stroke, and coronary revascularization; the corresponding mean NNT were 37, 319, and 107, respectively. Evolocumab positively affected MACE, myocardial infarction, stroke, and coronary revascularization; the corresponding mean NNT were 32, 78, 267, and 65, respectively. The effects of alirocumab or evolocumab on all-cause mortality and cardiovascular mortality were not statistically significant.”
The Physician’s Academy of Cardiovascular Education summarizes the effect as well [31]:
- PCSK9is reduced the risk of heart attack (RR: 0.81) and stroke (0.74) compared with control but not the risk of all-cause mortality (0.95) or CV mortality (0.95).
So the conclusions seem clear – PCSK9 inhibitors work for CVD, but not for overall mortality, perhaps because of some of the negatives of lowering cholesterol.
Can PCSK9 inhibitors reduce the overall death rate? Right now the clinical evidence doesn’t show whether it does or doesn’t. We’d need to wait for longer-term studies, since there was only a 1.5 yr follow up in the study listed.
However, if I were a betting man, I would guess that clinical trials will eventually show a change in overall mortality given the mendelian randomization studies and also because they bring LDL down quite dramatically (70%), without any significant side effects, as opposed to many other cholesterol lowering drugs.
Cholesterol Absorption Inhibitors
Next, let’s look at ezetimibe, which has a different mechanism of action than statins, PCSK9 inhibitors and bile acid sequestrants.
The Cochrane Review summarizes the research as follows [32]:
“Moderate‐ to high‐quality evidence suggests that ezetimibe has modest beneficial effects on the risk of CVD endpoints, primarily driven by a reduction in non‐fatal MI and non‐fatal stroke, but it has little or no effect on clinical fatal endpoints.”
The Physician’s Academy of Cardiovascular Education summarizes the effect as well [31]:
- Ezetimibe reduced the risk of non-fatal heart attack (RR: 0.87) and non-fatal stroke (0.82) compared with control but not the risk of all-cause mortality (0.99) or CV mortality (0.97).
Similar to PCSK9 inhibitors, ezetimibe seems to lower some types of CVD, but not overall death risk.
Niacin
Niacin used to be popular for lowering cholesterol, but has fallen out of favor because it didn’t lower overall mortality like statins do. In addition, with the introduction of statins, it didn’t seem to lower CVD risk more than statins alone.
However, as a monotherapy, it seems to help lower cardiovascular disease [33], as one might expect.
However, niacin comes with potential downsides, including negative effects on liver function, thyroid hormones, homocysteine, insulin resistance, and methylation. All of these can increase the risk of cardiovascular disease and overall death risk. Not to mention the negative effects of lower LDL.
This could be why there seems to be a positive effect for cardiovascular disease risk if someone doesn’t take a statin. After cutting cholesterol by 30-40% with statins, there are diminishing returns by cutting it another 10-20% with niacin, and the negative effects of high dose niacin may start to counteract the positive ones.
Fibrates
Fibrates are a class of drugs that reduce cholesterol in a unique pathway (PPARa).
According to a Cochrane review:
“Moderate‐quality evidence suggests a risk reduction of 16% with fibrate therapy for the combined outcome of death due to cardiovascular disease, heart attack, or stroke….but there is low‐quality evidence for no risk reduction for overall mortality or death from non‐CVD with fibrates.”
The same story seems to play out – reduced risk of CVD, but not reduced risk of death from all causes, based on the evidence available.
Bempedoic Acid
Bempedoic acid has a unique mechanism of action by inhibiting ATP citrate lyase, which is involved in the liver’s biosynthesis of cholesterol. Therefore, it stops the liver from producing cholesterol rather than having systemic effects like statins have – and therefore should have less side effects.
In a meta analysis where the longest follow up was 1 year, Bempedoic acid showed beneficial trends for non-fatal heart attacks and was associated with a lower risk of new-onset or worsening of diabetes, but had some side effects such as higher risk of gout, muscular disorders, and worsening kidney function [34].
The problem with this study is the very short follow ups and how long it takes for lowering LDL to have a big impact on cardiovascular disease. But even so, it did show some impact on lowering heart attack risk.
Bile acid sequestrants
The few small studies on bile acid sequestrants seem to show a similar trend of lower coronary artery disease risk [35], although I haven’t found larger studies done on overall mortality, so it’s difficult to make conclusions here.
In the same study, they did a Mendelian Randomization study by looking at SNP that naturally increases bile acid sequestration, and also found a lower coronary artery disease risk, which strengthens the clinical trial [35].
There are more confounding variables with bile acid sequestrants – they can lower thyroid hormones and increase triglycerides, both of which can increase CVD risk.
However, from the limited data that is out there, it seems like this class of drug fits into the same pattern as the other class of drugs: there is probably some reduction in CVD, but no evidence of lower overall mortality.
Plant-Based, Vegan & Vegetarian Diets
Plant-based diets are the main dietary method to reduce cholesterol. I don’t think anyone (besides maybe flat earthers) denies that it works for this goal – even carnivores won’t deny this. It’s pretty clear that plant-based diets lower LDL cholesterol. I’ve seen it on myself, on clients, in anecdotes, in studies, etc… I don’t hear much denialism about this.
Now let’s look at what happens to people on such diets.
A meta-analysis in 2022 of cohort studies of vegans and vegetarians found that CVD risk declined. This is not too surprising.
It concluded that “The associations between vegetarian diets and CVD and Ischemic heart disease were considered probably causal using WCRF criteria.” [36]
Now, what do you think these diets do to the overall risk of death?
When looking at overall mortality, a large meta-analysis concludes [36]:
“Conversely, healthful plant-based diets were associated with decreased CVD incidence (HR: 0.87), but not mortality.”
Another study finds similarly [37]:
“We found no evidence that following a vegetarian diet, semi-vegetarian diet or a pesco-vegetarian diet has an independent protective effect on all-cause mortality.”
So there you go. We see the same thing as the drug studies. You lower LDL cholesterol, you reduce the risk of some form of CVD, but you don’t decrease the overall risk of death.
I’ve seen some studies come to different conclusions such that plant-based diets does decrease mortality, but when you take a broad view of all the published studies, you get similar conclusions that it doesn’t.
And if there is an association that it does lower overall mortality, I wouldn’t trust it because of the healthy user bias, which is quite powerful. However, even with the healthy user bias, we still don’t see lower overall mortality in most studies.
Mendelian Randomizations (MR): A Game Changer in Causation Research
Mendelian randomization (MR), in very simple terms, is a research method used to determine whether a specific factor (such as a lifestyle habit or a biological trait) might have a causal effect on an outcome (such as a disease or a health condition) by using genetic information.
This method is based on the principle of Mendelian inheritance, which states that genes are passed down from parents to their offspring in a random manner. Because of this random inheritance, genetic variations are less likely to be influenced by external factors, such as lifestyle or environment, which can sometimes confuse or bias the results of observational studies.
Mendelian randomization uses these genetic variations as “natural experiments” to help researchers understand if there is a causal relationship between a particular factor and an outcome. For example, if researchers want to know if high cholesterol causes heart disease, they can compare the health outcomes of people with genetic variations that lead to naturally higher or lower cholesterol levels to those without such variations.
In essence, Mendelian randomization is a technique that utilizes the random assortment of genes to help determine if a specific factor might cause a particular outcome, reducing the potential for confounding factors that often occur in observational studies.
What Do Mendelian Randomization (MR) Studies Say?
Mendelian randomization studies consistently show that LDL cholesterol is a causal factor for heart disease [38].
These studies also show that higher LDL-C causally reduces lifespan and longevity [39]. Studies also show that ApoB causally reduces lifespan [40].
A 1-standard deviation increase in genetically proxied LDL-c was associated with 1.2 years lower lifespan. It also showed that approximately 42% of the causal effect of LDL-c on lifespan is mediated through pathways independent of CAD and ischaemic stroke [39].
It’s also noteworthy that this was a general population that is not selected for increased cardiovascular risk. The study concludes that “there is likely to be a net lifespan benefit of LDL-c lowering therapies, particularly for PCSK9 inhibitor” [39].
Now, you may ask, what about the drug studies that don’t show any change in longevity? These drugs are given when people are older generally (62 is the average age to start a statin). These genetic variants people are born with, and lipids cause damage very slowly from the day you’re born.
So let’s assume that it’s actually neutral from a longevity standpoint to have relatively higher cholesterol after the age of 65, from the age of 0-65, you still accumulated damage to your arteries from lipids.
So it would make sense that lipids can cause a higher death risk overall when looked at through an MR study.
A specific MR study from 2021 concludes that out of all of the measures, ApoB is the most significant causative factor, and that LDL-C is mainly a good proxy for ApoB [41].
It concludes that “ApoB, representing the total number of hepatic-derived lipoprotein particles, is the primary lipid determinant of cardiovascular disease.”
A more recent study published in December 2022 concludes that non-HDL cholesterol is more predictive than ApoB [42].
The study concludes: “These variants allowed us to show that the causal mechanism underlying the relationship between ApoB particles and coronary artery disease is more associated with non-HDL-C than ApoB particle concentration.”
It concluded this because adding effects on non-HDL-C into a model that already included those on ApoB significantly improved the genetically predicted coronary artery disease effects, while adding ApoB into the model including non-HDL-C did not [42].
LDL-C vs LDL-P vs APOB vs non HDL-C: Which one is the best causal marker for cardiovascular disease?
LDL-C refers to the level of LDL cholesterol in the blood.
LDL-P refers to the number of LDL particles in the blood, which is a more accurate measure of the risk for heart disease than just measuring LDL-C levels.
APOB refers to a specific protein found on LDL particles. Measuring the levels of ApoB can provide a more accurate assessment of the number of LDL particles in the blood and the risk for heart disease than just measuring LDL-C levels.
People criticize LDL cholesterol as not a causative factor to cardiovascular disease, but a correlative factor, mainly correlating with ApoB levels, which is the real contributor to cardiovascular disease.
As mentioned, a more recent study published in December 2022 concludes that actually, non-HDL cholesterol is more predictive than ApoB [42].
The jury isn’t completely out on what is the most causative or predictive – if ApoB is better than non HDL-C, or total LDL particles, or small dense LDL particles, LP(a), or oxidized LDL, etc…
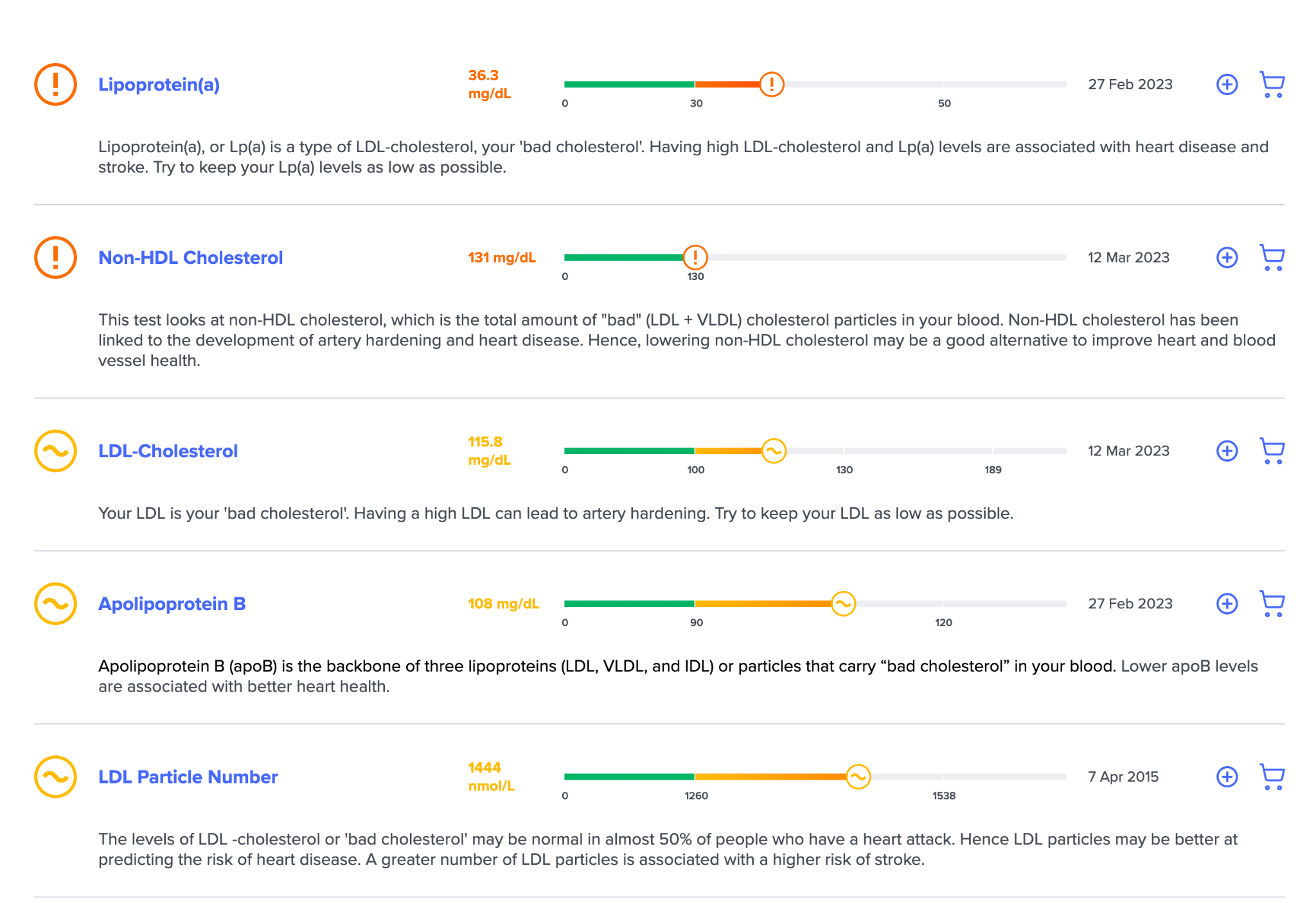
I have personally started to focus more on non HDL-C because it’s a cheap biomarker to track. I also track ApoB, LP(a), and LDL-P when I can. But keep in mind that LDL-C or non HDL-C will usually correlate quite closely with all of these. I recommend checking all of these biomarkers – at least once.
Prior Beliefs Lead to Dogma
Gurus who claim that cholesterol doesn’t have anything to do with heart disease, in almost 100% of the cases, are proponents of low-carb and high-fat diets, which tend to increase cholesterol.
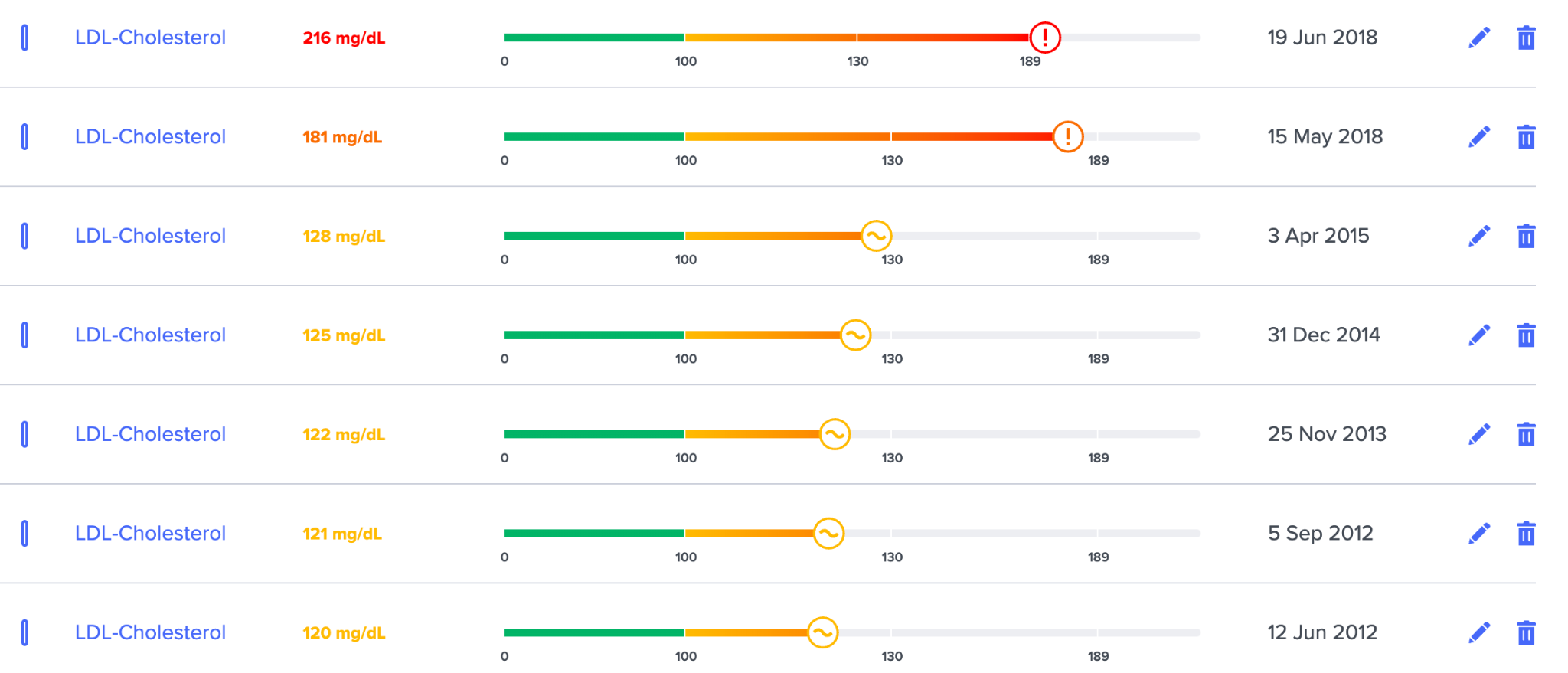
To be clear, I follow a paleo-type diet and am sympathetic to this diet overall. In addition, my own cholesterol has almost doubled after I implemented the diet. You can see the more carnivore I got, the more my LDL spikes, going from 120 to 216.
Saturated fats increase cholesterol in most people, and you can clearly see that I am one of those people. Studies show this, and I see it time and again with most people tested.
Once people get converted to the low-carb viewpoint, they then “look closely” at the cholesterol question and conclude what they wanted to conclude:
That lower carb, higher fat diets are perfect in every way and that if there’s a negative effect from studies, it’s because the studies are flawed. There simply can’t be any negatives or increased risks to the perfect diets that these people are proposing.
Before you attack me, be mindful that I am still on a high animal food diet!
But I also realize that in reality, the body is complex. There are pros and cons to every diet, and it’s a matter of what goals you are trying to promote and your genetics!
If a low-carb diet helps you lose weight or lower inflammation or lower triglycerides or blood glucose, even if it increases LDL cholesterol, it could still be a net positive – even for cardiovascular disease.
This idea that you can be doing something mostly good but that is also harming you in a specific way causes too much cognitive dissonance for most people.
But if you are trying to be optimal, then the proper way to think is – well I get XYZ benefits from low carb, but how do I lower the negatives from it? And making sure your lipids are in the optimal range is one way to do that.
Conclusions
What I conclude from the entirety of the evidence is that lipoproteins causally increase the risk for cardiovascular disease.
What also seems to be true is that reducing LDL cholesterol doesn’t reduce the risk of overall mortality unless it’s very high. There seems to be a sweet spot, most likely because of the benefits of LDL when people are older and start the medications.
Peter Attia’s philosophy is that CVD is such a big factor in mortality that if you reduce CVD risk it’s hard to imagine that you wouldn’t live longer. For himself, he says all the males in his family except his father died from heart disease at a young age, so he is rightly concerned about his genetic risk.
It’s a logical approach for him.
I think no one should have a very high non HDL cholesterol or ApoB if you want maximal longevity.
But then the question is how low should you go? This is where a more nuanced approach may be necessary.
Given the state of evidence, I think a common sense approach is as follows:
If you are at high genetic risk of CVD, it makes sense to do everything to reduce your risk as much as possible.
How do you know if you are at high risk (assuming you haven’t had a CVD event in the past)?
- Look at your genetic risk in SelfDecode
- Look at your lifestyle/environmental risk in SelfDecode
- Upload all relevant labs related to cardiovascular health in SelfDecode
Based on these variables, you will get a pretty good picture of what your risk is. If you’re at high risk based on these, then you will want to take action to get your lipids to an optimal range.
Next Steps
The first thing you should be doing is checking your genetically predicted risk for cardiovascular problems, particularly atherosclerosis.
In SelfDecode, you can see your genetically predicted results for a variety of predispositions that can help determine the best way to act.
1) Check Your Genetic Predisposition to Cardiovascular Disease
It’s known that people can have high cholesterol levels and ApoB, and still never get a cardiovascular event at an old age.
Genetics can help you figure out some missing pieces of the puzzle.
50% of the developed world dies from atherosclerosis or hardening of the arteries.
SelfDecode allows you to check what your risk profile is for artery hardening, which is the single most important factor for longevity, and certainly cardiovascular disease.
In addition, you can look at your risks for coronary artery disease, strokes, heart attack, deep vein thrombosis, heart failure, atrial fibrillation, and other cardiovascular diseases.
The most advanced medical centers, such as Stanford, are already using advanced polygenic risk scoring to see the genetic risk of patients for CVD and this will spread more widely in the future, but it’s still considered the cutting edge.
If you’re at high risk for these issues, then you should be even more concerned about having cardiovascular disease markers that are out of the optimal range – including ApoB and non HDL-C.
2) Check Your Labs & Environmental Risk
In the SelfDecode reports, you also can see and upload all of the markers that are relevant to a given risk so that you can not only see your genetic risk, but also the risks from your labs.
In addition, there are questionnaires that allow you to see your lifestyle risk.
The combination of these 3 things will help determine how much action you should be taking:
- Genetic predisposition
- Labs
- Lifestyle risk
SelfDecode also allows you to see a variety of lab markers and if they are predisposed to be out of the optimal range based on your genetics. You can then do the actual blood test to see what your result is!
In my case, it showed that my homocysteine, ApoB and blood pressure are predisposed to be high, and they all were without me taking action! I’ve recently gotten them all in the optimal range. ApoB was the last holdout.
3) Take Preventative Action
Based on these 3 areas, you can get an overall idea of what your risk is like and how aggressive you should be.
All SelfDecode reports and markers come attached with recommendations on how to improve that specific topic, including improving cardiovascular health and delaying CVD.
You can build a custom action plan with the recommendations from reports and also see how your health score will improve based on these additions.
All of the recommendations have double blind placebo controlled clinical trials attached to them.
4) Connect With Your Healthcare Practitioner
If you’re unsure of things, it makes sense to connect with your doctor. SelfDecode allows you to share all of your genetic information, environmental risks and lab tests directly with your healthcare practitioner!
They can easily search and find any lab and receive files and messages in a fully HIPAA & GDPR compliant software.
The practitioner can sign up for free to look at your results!
What Does Joe Do?
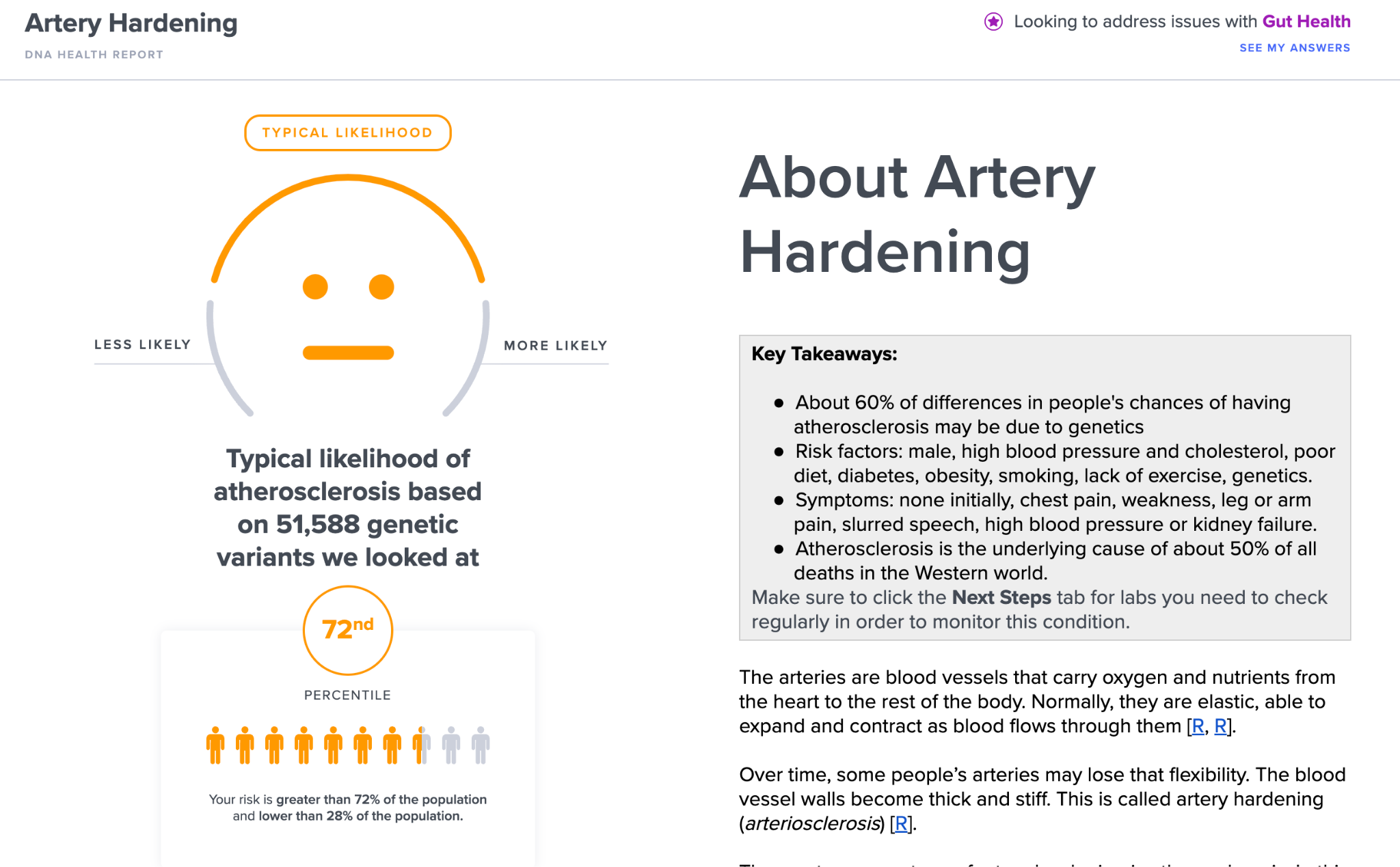
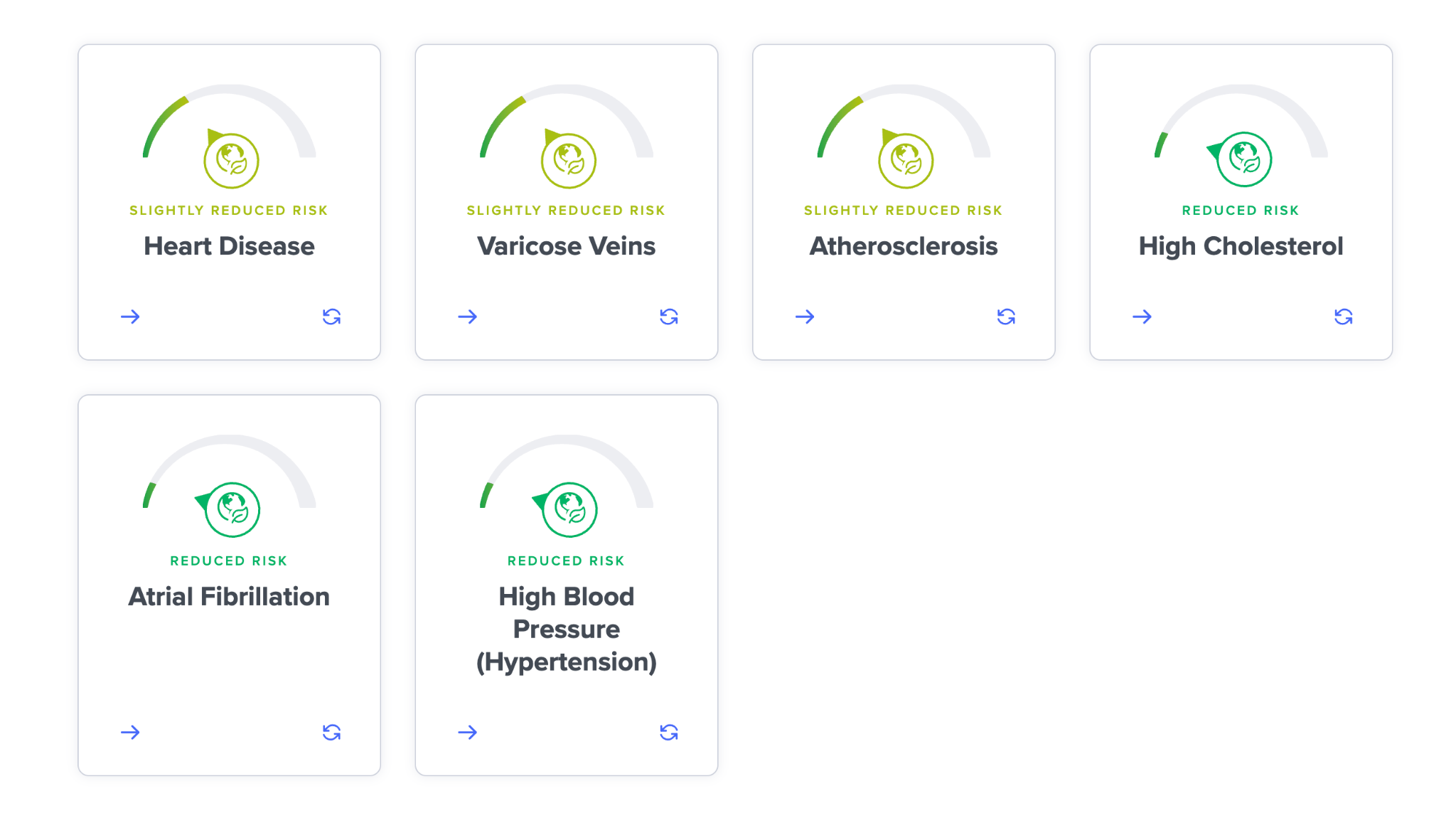
My results are in the 72nd percentile for atherosclerosis, which is higher than average but not too bad. We consider the 80th percentile or higher to be “high”.
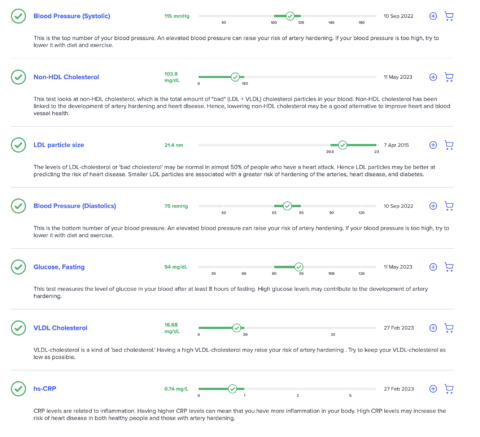
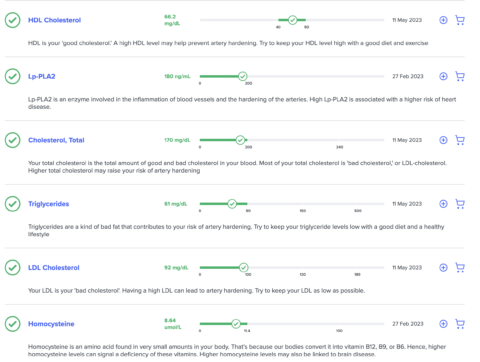
I’ve looked at all of my non-lipid results, and they are all in the optimal range for cardiovascular disease, per SelfDecode. These include insulin, homocysteine, hs-CRP and others.
When looking at the lipid-based labs connected to the report, it’s a mixed bag.
I have various lipids that are not in the optimal range, and I’ve been working on getting them in the optimal range with the suggestions laid out in SelfDecode.
I’ve recently brought all of my cardiovascular markers that I tested in the optimal range. I haven’t checked my ApoB and LP(a) since I brought my LDL down, because they aren’t as readily available in Israel. However, they track LDL-C close enough that I assume they are in the optimal range at this point.
Next, when I look at my lifestyle risk based on the SelfDecode questionnaire I filled out, I have lower environmental & lifestyle risk for cardiovascular disease:
Taking all of this information together, I’ve set for myself goals that I think are reasonable and provide the best risk/reward for me.
I think getting my APOB under 90 and my non HDL-C under 110 is a proper goal for me. I’d like my LP(a) to be under 20.
I believe that in the next blood test, I will accomplish these goals.
My conclusions are as follows:
- If you are at high genetic risk for cardiovascular disease, then you should take preventative measures.
- Make sure to check all of your biomarkers related to cardiovascular disease risk. Many are more important than LDL-C.
- Cholesterol and Lipoproteins are a factor in heart disease, and it shouldn’t be too high, but also you don’t have to go crazy with it and get it so low.
- If you can’t make heads from tails, connect your SelfDecode results with a practitioner.